
AnswerBasic amine formula is A-NH2 A-NHR or A-NR2. Amines are the chemical molecules which have amine -NH 2 group attached to its carbon atoms.
Humates actually hold many times their weight in.
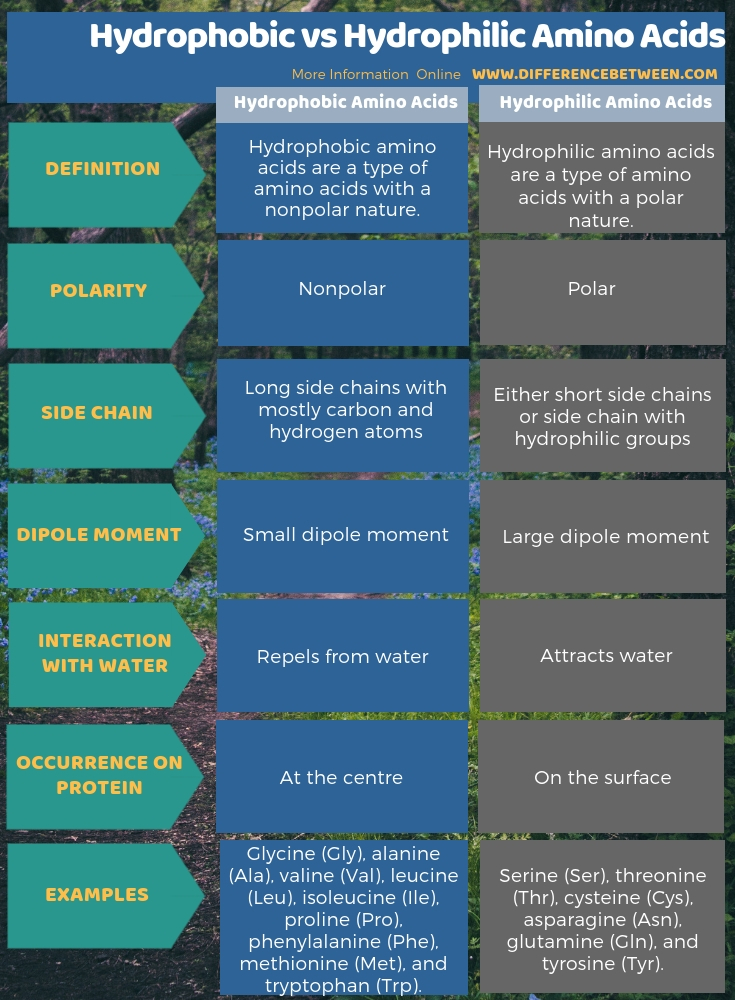
Amine hydrophilic or hydrophobic. Hydrophobic amino acids are a type of amino acids which have a nonpolar nature while hydrophilic amino acids are a type of amino acids in which have a polar nature. Hence this is the key difference between hydrophobic and hydrophilic amino acids. The three major classes of hormones comprise of polypeptides steroids and amines.
Amines are the chemical molecules which have amine -NH 2 group attached to its carbon atoms. The major amines that synthesised in the animals are act as hormones or signalling molecules. For example dopamine norepinephrine and epinephrine are called biogenic.
A hydrophilic membrane is the ideal selection for this type of application as continuous flow of 09 Normal Saline is extremely important when treating a severely dehydrated patient. Conversely hydrophobic materials are water hating or repelling therefore hydrophobic filters are typically used in gas filtration processes. The hydrophobicity of a filter allows non-polar fluids like air to interact more.
Most hydrophilic polymers are grouped by the chemistry of their structure. For example acrylics include acrylic acid acrylamide and maleic anhydride polymers and copolymers. Are amines hydrophilic or hydrophobic messengers.
How does this affect their release transport and signaling. Expert Answer 100 1 rating Previous question Next question. When amine was mixed with hydrophilic solvent H 2 O the activation energy of amine NMBZA to react with CO 2 was found to be 2766 kJmol 1 and decreased to 1037 kJmol 1 when mixed with hydrophobic solvent ethereal hydrophobic solvent.
The activation energy of hydrophobic solvent was found to increase by adding an alcohol to the solvent MePhOH ethereal hydrophobic. AnswerBasic amine formula is A-NH2 A-NHR or A-NR2. In which A is an alkyl or aryl group singly bonded may be substituted Primary will have no R group like phenylamine C6H5-NH2Secondary.
Is a hydrophobic silica prepared by the chemical reaction of hydrophilic fumed silica reactive silanes eg chlorosilanes or hexamethyldisilazane amine oxide. It is hydrophobic water repellent and can not be dispersed in water. The beneficial organic acids created by humic substances are extremely hydrophilic or having a strong affinity for water.
The polycyclic functional groups of humic acid molecules hold or chelate water. Humates actually hold many times their weight in. Hydrophobic and hydrophilic are frequently used descriptors of surfaces.
A surface is hydrophobic if it tends not to adsorb water or be wetted by water. A sur-face is hydrophilic if it tends to adsorb water or be wet-ted by water. More particularly the terms describe the interaction of the boundary layer of a solid phase with liquid or vapor water.
Obviously the hydrophilic UiO-66-NH 2 can be effectively converted to hydrophobic ones through the grafting of branched hydrocarbon chains. This strategy is versatile for amine-based hydrophilic MOFs conversion to hydrophobic. Cr-MIL-101-NH 2 as an example was also functionalized with branched hydrocarbon chains.
Hydrophobicity scales are values that define the relative hydrophobicity or hydrophilicity of amino acid residues. The more positive the value the more hydrophobic are the amino acids located in that region of the protein. These scales are commonly used to predict the transmembrane alpha-helices of membrane proteinsWhen consecutively measuring amino acids of a protein changes in value.
The nine hydrophobic amino acids are alanine Ala glycine Gly valine Val leucine Leu isoleucine Ile phenylalanine Phe proline Pro methionine Met and tryptophan Trp. The nine hydrophilic amino acids are listed below with the remaining two amino acids tyrosine Tyr and cysteine Cys defying categorization at this time. Amino acids are organic compounds that contain amino NH 2 and carboxyl COOH functional groups along with a side chain R group specific to each amino acid.
The key elements of an amino acid are carbon C hydrogen H oxygen O and nitrogen N although other elements are found in the side chains of certain amino acids. Each of the 20 most common amino acids has its specific chemical characteristics and its unique role in protein structure and function. For example based on the propensity of the side chain to be in contact with water amino acids can be classified as hydrophobic low propensity to be in contact with water polar and charged energetically favorable contacts with water.
Upon adding the aqueous amine solution and mixing a phase split was produced between a hydrophilic phase consisting of the water and ammonium carboxylate salts of the hydrophilic NaDES-Y and a hydrophobic phase made by sun ower oil. Prior to the fourth part of the work microalgal biomass of Scenedesmus dimorphus UTEX 1237 was cultivated in our. Tertiary amine - Hydrophilic Hydrophobic Penetration of skin.
Selection of the amino acids is based on the types of interactions that are possible with the particular functional group. With the tertiary amine it is essential to consider the ionization of this functional group prior to pairing with an amino acid. For the ion.
Hydrophilic or water-soluble hormones are unable to diffuse through the lipid bilayer of the cell membrane and must therefore pass on their message to a receptor located at the surface of the cell. Except for thyroid hormones which are lipid-soluble all amino acidderived hormones bind to cell membrane receptors that are located at least.