Each amino acid is a nitrogenous compound having both an acidic carboxyl COOH and a basic amino NH2 group. Of the twenty amino acids that make up proteins six of them have acid or base R-groups.

Amino acids are organic compounds that combine to form proteins.
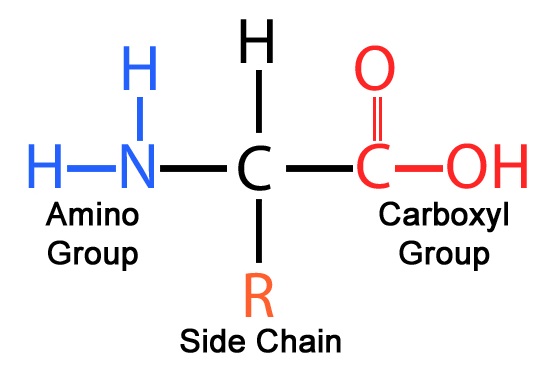
Amino acid group structure. Generally amino acids have the following structural properties. A carbon the alpha carbon A hydrogen atom H A Carboxyl group -COOH An Amino group -NH 2 A variable group or R group. Amino acids are organic compounds that combine to form proteins.
The general formula of an amino acid is R-CH NH 2-COOH. Amino acids are known to contain amine and carboxyl functional groups. They also contain a side chain that is made up of an R-group where R can denote any alkyl or aryl group.
An amino acid is an organic molecule that is made up of a basic amino group NH 2 an acidic carboxyl group COOH and an organic R group or side chain that is unique to each amino acid. The term amino acid is short for α-amino alpha-amino carboxylic acid. Each amino acid is a nitrogenous compound having both an acidic carboxyl COOH and a basic amino NH2 group.
R stands for the side chains that are different for each amino acid. R can be as simple as a hydrogen atom H or a methyl group CH3 or a more complex structure. The first carbon is the part of the carboxyl group.
There are 20 naturally occurring amino acids and all have common structural features an amino group -NH3 a carboxylate -COO- group and a hydrogen-bonded to the same carbon atom. They differ from each other in their side-chain called R group. Each amino acid has 4 different groups attached to α- carbon.
These 4 groups are. An amino acid is a carboxylic acid-containing an aliphatic primary amino group in the α position to the carboxyl group and with a characteristic stereochemistry. Proteins are biosynthesized from 20 amino acids in a system involving strict genetic control.
Thus amino acids are the basic unit of. Arg Lys His pH of side group is 6 Polar Uncharged. Ser Thr Cys Tyr Gln Aln Blosum-62 Substitution Matrix The Blosum-62 substitution matrix shownon the next page describes the degree to which specific amino acids are substituted for other amino acids.
The matrix was derived by examining substitutions that occur. All amino acids have the same basic structure which is shown in Figure 21. At the center of each amino acid is a carbon called the α carbon and attached to it are four groups - a hydrogen an α- carboxyl group an α-amine group and an R-group sometimes referred to as a side chain.
Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. This carbon is designated as the α-carbon alpha-carbon. Amino acids differ from each other with respect to their side chains which are referred to as R groups.
Amino acids are the monomers that make up proteins. Each amino acid has the same fundamental structure which consists of a central carbon atom also known as the alpha α carbon bonded to an amino group NH 2 a carboxyl group COOH and to a hydrogen atom. Each amino acid has at least one amine and one acid functional group as the name implies.
The different properties result from variations in the structures of different R groups. The R group is often referred to as the amino acid side chain. Amino acidsare organic compounds which contain both an amino group and a carboxyl group.
They are distinguished by the attached functional group R. Of the twenty amino acids that make up proteins six of them have acid or base R-groups. Compare with the simplest of the amino acids glycine which has only H as an R-group.
All amino acids have a central carbon atom surrounded by a hydrogen atom a carboxyl group COOH an amino group NH2 and an R-group. It is the R-group or side chain that differs between the 20. General Structure of Common Amino Acids General structure of amino acidsgroup and a variable side chain Side chain determines.
Protein folding binding to specific ligand and interaction with its environment Amino acids consists of a constantCOOHR is side chain At neutral pH H 2 N-.