Circular dichroism CD is a valuable technique for examining the protein confor-mation in solution and assess information on the secondary and tertiary protein structure. However the value of many studies using CD is compromised either by inappropriate experimental design or by lack of attention to key.
Analysis of circular dichroism spectra of proteins provides information about protein secondary structure.
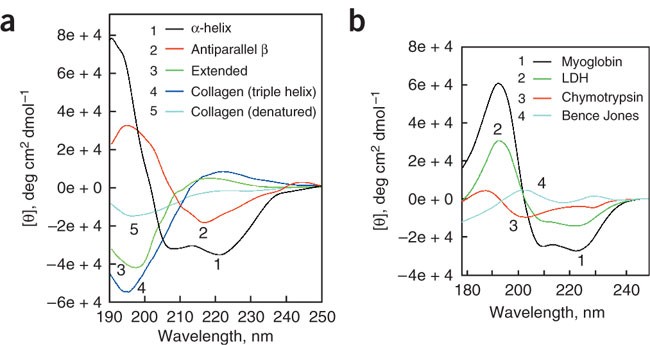
Computation and analysis of protein circular dichroism spectra. This chapter presents computation and analysis of protein circular dichroism CD spectra. The origins of electronic CD in proteins theoretical methods for computing protein CD and empirical analysis of CD for estimating structural composition of proteins are described. The phenomenon of CD involves the absorption of light and it can be considered as a special type of absorption spectroscopy.
The CD spectra of proteins. Computation and analysis of protein circular dichroism spectra. Analysis of protein secondary structure by circular dichroism spectroscopy indicated that the amino-terminal domain of PvTIP31 is generally unstructured and.
Computation and Analysis of Protein Circular Dichr oism Spectra. Introduction Circular dichroism CD is the most widely used form of chiroptical spectroscopy spectroscopic techniques that utilize the differential interaction of molecules with left and rightcircularly polarized light. Computation and Analysis of Protein Circular Dichroism Spectra.
Methods in Enzymology 2004. Circular dichroism CD which is a consequence of molecular chirality is an important method for the investigation of protein structure and structural changes during interactions with ligands mutations and folding. The development of computational methods allows powerful insight to be provided into the mechanisms of generation of CD spectra in complex systems as proteins and to explain.
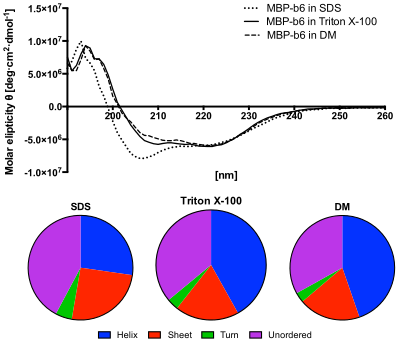
Circular dichroism spectra of HoloMyoglobin Mb as a function of temperature. Circular dichroism measurements were recorded using a Jasco J. Circular dichroism CD is an excellent method for rapidly evaluating the secondary structure folding and binding properties of proteins.
Briefly CD is defined as the unequal absorption of. Computation and Analysis of Protein Circular Dichroism Spectra Methods Enzymol. 383 2004 pp.
318 - 351 Article Download PDF View Record in Scopus Google Scholar. Analysis of circular dichroism spectra of proteins provides information about protein secondary structure. Analytical methods developed for such an analysis use structures and spectra of a set of reference proteins.
The reference protein sets currently in use include soluble proteins with a wide range of secondary struc-. Circular dichroism CD is being increasingly recognised as a valuable technique for examining the structure of proteins in solution. However the value of many studies using CD is compromised either by inappropriate experimental design or by lack of attention to key.
Circular dichroism CD is a valuable technique for examining the protein confor-mation in solution and assess information on the secondary and tertiary protein structure. The near UV wavelength range 350 to 250 nm is sensitive to changes or differences in tertiary. To this end we introduce a new computational method to calculate the electronic circular dichroism spectra of proteins from a structural model or ensemble using the average secondary structure composition and a precalculated set of basis spectra.
The method is designed for model validation to estimate the error of a given protein structural model based on the measured CD spectrum. Circular dichroism CD spectroscopy has been a valuable method for the analysis of protein secondary structures for many years. With the advent of synchrotron radiation circular dichroism SRCD and improvements in instrumentation for conventional CD lower wavelength data are obtainable and the information content of the spectra increased.
In contrast to the analysis of protein CD spectra to extract information perhaps on secondary structure content through the application of statistical or machine learning methods which is an active field in its own right the focus of DichroCalc and its applications usually start with the generation of atomistic models or hypotheses of structure andor conformational change followed by. PDF Circular Dichroism CD Analysis of Protein Training Poster. Master Course of Methods in Biochemistry Find read and cite all the research you need on ResearchGate.
Experimental and computational aspects of the quantitative analysis of vibrational circular dichroism VCD of proteins are discussed. Experimentally the effect of spectral resolution sample concentration cell selection and spectral normalization effects are considered. The influence of random intensity variations on the results of quantitative analysis of amide I VCD are shown to be minor up to a 15 variation in spectral intensity.
A computational algorithm based on factor analysis. Circular dichroism spectroscopy is a structural biology technique frequently applied to determine the secondary structure composition of soluble proteins. Our recently introduced computational analysis package SESCA aids the interpretation of protein circular dichroism spectra and enables the validation of proposed corresponding structural models.