
It was followed by vegetable oil then soya bean oil then olive oil and margarine had the lowest saponification value. The saponification value is a measure of the free and esterified acids present in fats and fatty acids.
Determination of saponification value of fatoils Objective.
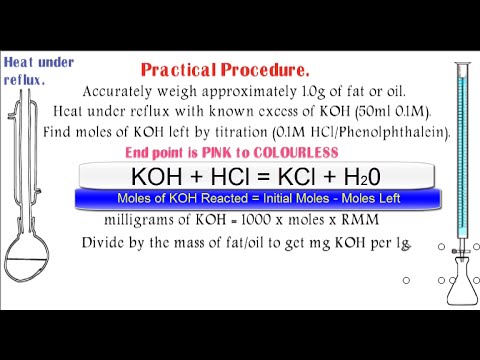
Determination of saponification value of oil. The saponification value of an oil is defined as the number of milligrams of potassium hydroxide required to neutralise the fatty acids resulting from the complete hydrolysis of 1 g of the sample. Investigatory Project on Determination of Saponification Value of Oil 1. What are oils and fats chemically.
Oils and fats are glycerides of higher fatty acids. Give the name and formulae of three important fatty acids found in oils and fats. Palmitic acid - C15H31COOH.
Saponification is the hydrolysis of fats or oils under basic conditions to afford glycerol and the salt of the corresponding fatty acid. Saponification literally means soap making. It is important to the industrial user to know the amount of free fatty acid present since.
Saponification value is defined as the amount of potassium hydroxide KOH in milligrams required to saponify one gram of fat or oil. It is a very important. Determination of saponification value of fatoils Objective.
To Estimate the Saponification value of oils. Fats and oils are the principle stored forms of energy in many organisms. They are highly reduced compounds and are derivatives of fatty acids.
10 Calculate the saponification value using the formula. Saponification value or number of fat mg of KOH consumed by 1g of fat. Weight of KOH Normality.
The milligrams of KOH required saponifying 1 g of oil known as saponification value determination of saponification value accurately weighed quantity of oil is taken in a flask and 50 mL of 01 alcoholic KOH is added. The mixture is heated. The saponification value is the number of mg of potassium hydroxide required to neutralize the fatty acids resulting from the complete hydrolysis of 1 g of the substance.
In the procedure described a 50-mL burette should preferably be used for titration as in the blank titration the volume of. Saponification value is a measure of the content of ester linkages. It is determined by back titration of potassium oxide in the presence of phenolphthalein indicator with 05 N sulfuric or hydrochloric acid39 First a sample is mixed with 25 ml of alcoholic solution.
Using these values the saponification values of oils are determined. Saponification value 561 V2 - V1 strength of Hcl W Molecular weight of KOH 561 Weight of oil W ESTIMATION OF IODINE VALUE A standard solution 01N potassium dichromate was. Such a determined constant of saponification value is then sought and marked on the left hand side of the vertical scale SV.
Since this car rying line is common for both the saponification and neutralization values as a double-scale the plotted saponification value gives us the corresponding neutralization value. This document specifies a method for the determination of the saponification value of animal and vegetable fats and oils. The saponification value is a measure of the free and esterified acids present in fats and fatty acids.
The method is applicable to refined and crude vegetable and animal fats. The saponification value of the rancid extracted oil is negative this is because the titre value of the concentrated HCl was greater than the blank titre. It was followed by vegetable oil then soya bean oil then olive oil and margarine had the lowest saponification value.
ISO 36572013 specifies a method for the determination of the saponification value of animal and vegetable fats and oils. The saponification value is a measure of the free and esterified acids present in fats and fatty acids. The method is applicable to refined and crude vegetable and animal fats.
36 Determination of Refractive Index. According to IUPAC 2102 or ISO 6320. 37 Determination of Saponification Value SV According to IUPAC 2202 or ISO 3657.
38 Determination of Iodine Value IV Wijs-according to IUPAC 22051 ISO 3961. 1996 AOAC 99320 or AOCS Cd 1d-1992 97. 39 Determination of Unsaponifiable Matter.