The hydrolysis of pyrophosphate to two molecules of inorganic phosphate Pi reaction is highly energetically favorable and drives the other two reactions. At this wavelength the absorbance of the ester substrate was negligible.

An arginine-reactive colored reagent A max 340 nnm to label and measure the arginine amino acids or arginine-containing peptides by HPLC.
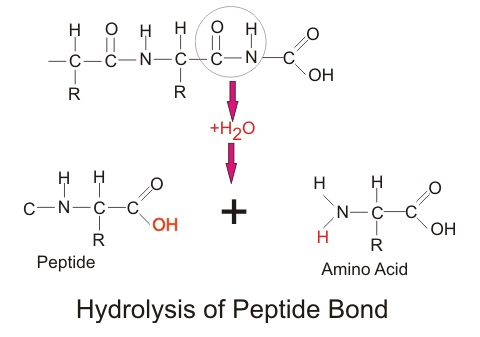
Hydrolysis reaction for amino acids. There is no single hydrolysis method that will effectively cleave all proteins to single amino acids completely and quantitatively. This is owing to the varying stability of the peptide bonds between the different amino acids and the amino acid side chains which are themselves susceptible to the reagents and conditions used to cleave the peptide bonds Table 1. Hydrolysis methods are used for liberate amino acids from protein substrate and quantitatively recover them in the hydrolysate.
Due to a large number of factors such as temperature time. There is no single hydrolysis method that will effectively cleave all proteins to single amino acids completely and quantitatively. This is owing to the varying stability of the peptide bonds between the different amino acids and the amino acid side chains which are themselves susceptible to the reagents and conditions used to cleave the peptide bonds see Table 1.
Amino Acid Peptide Linkage and Hydrolysis Reactions. If playback doesnt begin shortly try. The hydrolysis technology and reaction kinetics for amino acids production from fish proteins in subcritical water reactor without catalysts were investigated in a reactor with volume of 400 ml under the conditions of reaction temperature from 180320C pressure from 5-26 MPa and time from 5-60 min.
The quality and quantity of amino acids in hydrolysate were determined by. Add 500 iL of hydrolysis mixture to the reaction vessel Fig 1. Slowly purge the hydrolysis acid mixture in the reaction vessel and the minmert.
The anaerobic degradation of each amino acid that could be generated through the hydrolysis of sewage sludge was evaluated. Stickland reaction as an intermediate reaction between two kinds of amino acids was restricted in order to evaluate each amino acid. Changes in the chemical oxygen demand COD T-N NH4-N biogas and CH4 were analysed for the anaerobic digestion process.
The hydrolysis rates of the amino acid esters in the presence of CuL 1 and CuL 2 were measured with an initial slope method by monitoring the increase in the 400-nm absorption of the released NP. At this wavelength the absorbance of the ester substrate was negligible. The reaction solution was maintained at 298 01 K.
HEPES buffer pH 72 50 mM was used and the ionic. During acid hydrolysis to permit the amounts of these amino acids to be estimated quantitatively from the results of auto- matic amino acid analysis 1. Hence the determination of the number of residues of these amino acids is usually made either by analyzing for cysteic acid after performic acid oxidation of.
The quality and quantity of amino acids in hydrolysates were determined and 17 kinds of amino acids were obtained. Under the tested conditions the highest amino acid yield 325 mgg protein was reached at an initial substrate concentration of 10 gl a temperature of 250 C and a reaction time of 60 min. The acid-hydrolysis reaction with 6 M HCl results in the addition of water to each covalent peptide bond yielding the desired individual amino acids Figure 1.
However not all amino acids are completely recovered under hydrolysis by HCl. Some amino acids are hydrolyzed to their acid forms such as asparagine and glutamine which form aspartic acid and glutamic acid respectively. Total acid hydrolysis of the substituted peptide or protein yields a mixture of free amino acids plus the dansyl derivative of the N-ter.
The reagent l-dimethylaminonaphthalene-5-sulfonyl chloride dansyl chloride DNS-C1 reacts with the free amino groups of peptides and proteins as shown in Fig. The overall net reaction is. Amino Acid ATP tRNA Aminoacyl-tRNA AMP PP i The net reaction is energetically favorable only because the pyrophosphate PPi is later hydrolyzed.
The hydrolysis of pyrophosphate to two molecules of inorganic phosphate Pi reaction is highly energetically favorable and drives the other two reactions. A look at the reactions of Amino Acids with alkalis acids and alcohols About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features. An arginine-reactive colored reagent A max 340 nnm to label and measure the arginine amino acids or arginine-containing peptides by HPLC.
Hydrochloric Acid HCl Solution sequencing grade Constant-boiling 6 N HCl solution supplied in 1 mL ampules used to acid-hydrolyze proteins protein hydrolysis for HPLC amino acid composition analysis. To give dansyl amino acids according to the scheme. Dan-C1 H20 3- Dan-OH HC1 Dan-C1 amino acid 23- Dan-amino acid HC1.
The actual observed reaction rate ks in water solution is the sum of the rate of hydrolysis and that of reaction with amino acid. Kobs k HO k amino acid. Effects of amino acid structure ionic strength and magnesium ion concentration on rates of nonenzymic hydrolysis of aminoacyl transfer ribonucleic acid.
Biochemistry 1972 11 12 2321-2323. Peter Schofield Brian Mark Hoffman and Alexander Rich.