
In an incident on January 12 2006 at Texas AM University the pressure-relief devices of a tank of liquid nitrogen were malfunctioning and later sealed. Latent Heat of Vaporization of Nitrogen is 27928 kJmol.
Lower limit for calculation.
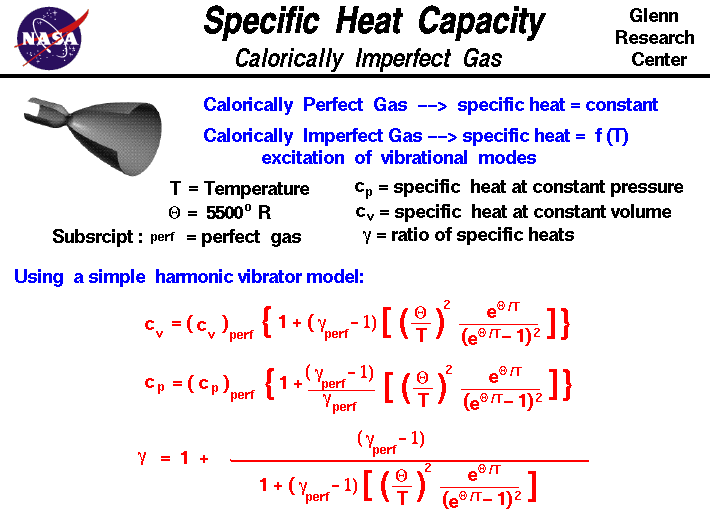
Specific heat ratio of nitrogen. Specific heat of Nitrogen Gas - N2 - at temperatures ranging 175 - 6000 K Isobaric specific heat Cp is used for substances in a constant pressure ΔP 0 system. Isochoric specific heat Cv is used for substances in a constant-volume isovolumetric or isometric closed system. Du cv dT 1 where.
Du change in internal energy kJkg cv specific heat for gas in a constant volume process kJkgK dT change in temperature K Specific heat cv varies with temperature but within moderate temperature changes the specific heat - cv - can be regarded as constant. Specific and molar heats of gaseous nitrogen at constant pressure. C p 02491 00000095t.
In the next table are given the molecular specific heats at constant volume and the ratio C p C v. Nitrogen Specific Heat Specific heat of Nitrogen is 104 Jg K. Because the liquid-to-gas expansion ratio of nitrogen is 1694 at 20 C 68 F a tremendous amount of force can be generated if liquid nitrogen is vaporized in an enclosed space.
In an incident on January 12 2006 at Texas AM University the pressure-relief devices of a tank of liquid nitrogen were malfunctioning and later sealed. Calculation of thermodynamic state variables of nitrogen in saturation state boiling curve. Lower limit for calculation.
-210 C 013 bar bar upper limit. -148 C 33 bar. BarMillibarMPak PaPascalN mm2kp cm2 atülb feet2psi lb inch2Torr mm Hginch Hgmm H2Oinch H2Ofeet H2O.
At 15 degrees celcius. Mixture in the ratio 051049. These were found to be.
11652 00003 14353 00003 12377 00001and 13587 00002 respectively. The small uncertainties in γ suggest a precise procedure while the discrepancy between experimental and accepted values indicates inaccuracy. If the constituents are taken to be perfect gases and the Gibbs-Dalton law holds what are the molecular weight and specific heat ratio of the mixture.
Mole wt 28 Specific Heat Ratio. 47 Isobaric Specific Heat Capacity of the Saturated Liquid Data for the isobaric specific heat capacity of the saturated liquid are represented by Equation 4747 The significance of as a representation of specific heat capacity is fully explained in Appendix B Section B1. Loge p pc—– 1 Tr—–b 11 Tr b21 Tr 32 b 31 Tr.
Molar heat capacity 29124 Jmol K both at 25 C N2. The Cp of nitrogen at amospheric pressure changes for about half a percent between 50 deg C 024895 and 150 deg C 025021 kcalkg hardly a change to be taken into account. For more thermophysical nitrogen properties the website recommended by CRG is excellent.
Specific heat of Nitrogen is 104 Jg K. Latent Heat of Fusion of Nitrogen is 03604 kJmol. Latent Heat of Vaporization of Nitrogen is 27928 kJmol.
The ratio of heat capacity at constant pressure to heat capacity at constant volume for the three gases. Nitrogen Carbon dioxide and Argon were estimated as 141 2 129 2 and 1673 5 by measurement of the speed of sound through the gas. Breathing Air is approximately 79 Nitrogen.
It is typically used as an inert gas in electrical systems in the chemical industry the food packing industry and the semi-conductor industry. The heat of dissociation of gaseous nitrogen tetroxide has been given by various observers as -13000 -13050 -12900 -13920 and -13132 calories respectively. The heat of formation of nitrogen tetroxide containing 9 per cent of NO2 from 2 N 4 O N2O4 is -3900 calories.