Photo decomposition is a chemical reaction in which a substance is broken down into simple substances by exposure to light photons. In general pyrolysis of organic.

DECOMPOSITION REACTION Those reactions in which a compound splits up into two or simpler substances are known as decomposition reactions.
What happens in a thermal decomposition reaction. Thermal decomposition is an example of an endothermic reaction a reaction that gains energy from the surroundings. This is why thermal energy must be supplied constantly for the reaction to keep. Thermal decomposition is a chemical reaction where heat causes one substance to break into two or more different substances.
The heat is used to break down the bonds holding the atoms of the original molecules together so the reaction usually consumes more heat energy than it releases. The chemical reactions are classified according to the processes there are many types such as The thermal decomposition reactions The substitution reactions The oxidation and reduction reactions. The thermal decomposition reactions are the reactions which involve the breaking up of the compounds by the effect of heat into its simple elements or simpler compounds than the original ones.
On further heating anhydrous ferrous sulphate decomposes to form ferric oxide Fe 2 O 3 sulphur dioxide SO 2 and sulphur trioxide SO 3. So the gas emitted smells like burning sulphur. In this reaction the single reactant FeSO 4 decomposes to form three different products.
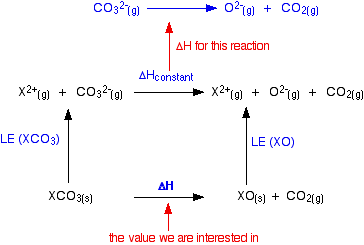
So the reaction is a decomposition reaction. Thermal decomposition of metal carbonates This experiment involves a comparison between the thermal stabilities of carbonates of reactive metals such as sodium and potassium and the carbonates of less reactive metals such as lead and copper Metal carbonates decompose when heated. Some carbonates are more reactive than others.
The thermal decomposition pyrolysis of urea to produce cyanuric acid CYA. 246-trihydroxy-135-triazine was discovered by Wöhler approximately 175 years ago. Thermal decomposition of calcium carbonate Calcium carbonate is strongly heated until it undergoes thermal decomposition to form calcium oxide and carbon dioxide.
The calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater. Bubbling carbon dioxide through this forms a milky suspension of calcium carbonate. The amount of each type of fraction obtained by fractional distillation does not usually match the amount of each fraction that is needed.
Crude oil often contains a greater quantity of heavier fractions than lighter fractions. Thermal DecompositionIn this video we cover What thermal decomposition is What happens to the atoms in this reaction Some examplesKS3 Sci. Photo decomposition is a chemical reaction in which a substance is broken down into simple substances by exposure to light photons.
Thermal decomposition reaction Thermolysis Decomposition of calcium carbonateCalcium carbonate lime stone decomposes into calcium oxide quick lime and carbon dioxide when heated. Quick lime is the major constituent of cement. Pyrolysis is the thermal decomposition of materials at elevated temperatures in an inert atmosphere.
It involves a change of chemical compositionThe word is coined from the Greek-derived elements pyro fire and lysis separating. Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.
In general pyrolysis of organic. Thermal decomposition of other amphiboles asbestos also confirmed IR spectra Fig. 13 where beyond the absence of OH bands can be seen clearly shifts to lower frequencies of the SiOSi bands.
The temperature range of non-isothermal heating was too low so cannot create well-crystallized products of the thermal decomposition of amphiboles. A thermal decomposition reaction can be defined as a decomposition reaction which is activated by thermal energy. In other words a thermal decomposition reaction requires energy to be supplied to the reactants in the form of heat.
Decomposition of Calcium Carbonate Decomposition of Calcium Carbonate is an example of thermal decomposition. Calcium carbonate is heated strongly until it undergoes thermal decomposition to form calcium oxide and carbon dioxide. The calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater.
Bubbling carbon dioxide through this forms a milky suspension of calcium carbonate. In thermogravimetric analysis TGA a sample is continually weighted while heating as an inert gas atmosphere is passed over it. Many solids undergo reactions that evolve gaseous byproducts.
In TGA these gaseous byproducts are removed and changes in the remaining mass of the sample are recorded. Three variations are commonly employed. Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries.
Calcium oxide is called lime or quick lime. It has many uses one is in the manufacture of cement. When a decomposition reaction is carried out by heating it is called thermal decomposition.
DECOMPOSITION REACTION Those reactions in which a compound splits up into two or simpler substances are known as decomposition reactions. The decomposition reactions are carried out by applying heat light or electricity. Heat light or electricity provide energy which breaks a compound into two or more simpler compounds.