Opacus PD630 and R. The strength of an alkali or acid depends.

Ammonia are dark blue in colour due to solvated metal ions and solvated electrons.
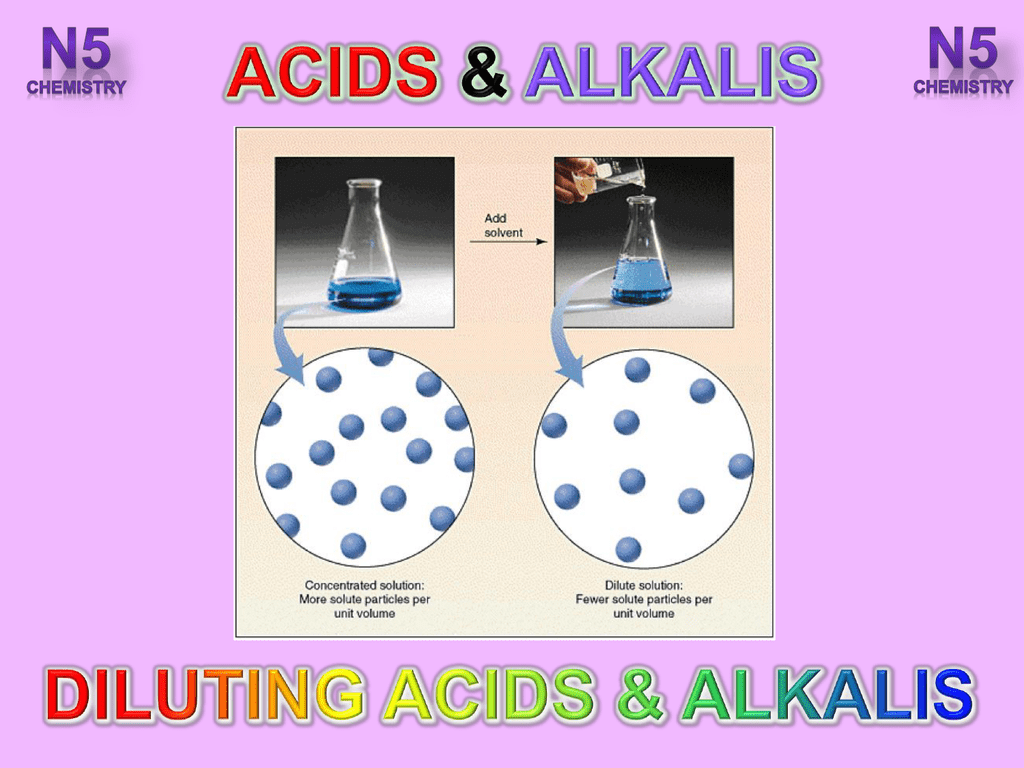
What is a dilute alkali. A dilute alkali pretreatment NaOH was used to remove lignin and some hemicelluloses as well as to efficiently increase the accessibility of enzymes to the cellulose in Amur silvergrass. When alkalis are diluted the pH goes down toward 7. A tenfold dilution of hydrochloric acid will change its pH by 1 pH unit as will a tenfold dilution of sodium hydroxide.
This is because the dilution of hydrochloric acid changes the concentration of hydrogen ions H by a factor of ten. Comparison of Dilute Acid Alkali and Biological Pretreatments for Reducing Sugar Production from Eucalyptus. Haojun Lu a Xinwen Zhang a Aimin Wu a Xiaomei Deng a Junli Ren b Fangong Kong c and Huiling Li abc.
The effects of chemical pretreatments dilute H 2 SO 4 dilute NaOH and NH 4 OH and biological pretreatments Coriolus versicolor and Daedalea quercina on. Alkalis and acids can be described as strong or weak. This does not mean the same as concentrated or dilute.
The strength of an alkali or acid depends. On how ionised it is in water. An alkali forms hydroxide ions OH - ions in water.
Hydrolysis using dilute alkali. This is the usual way of hydrolyzing esters. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution.
There are two advantages of doing this rather than using a dilute acid. The reactions are one-way rather than reversible and the products are easier to separate. The salt of a carboxylic acid.
Reaction of a metallic alkali base with a fat or. Oil to form soap. Formation of a white colloidal matter after the.
Addition of coconut oil in dilute alkali. A very dilute gas of atomic sodium about 1000 atoms per cubic cm about 16000 atoms per cubic inch is produced in Earths mesosphere altitude about 90 km 60 miles by ablation of meteors. Subsequent reaction of sodium with ozone and atomic oxygen produces excited sodium atoms that emit the light we see as the tail of a meteor as well as the more diffuse atmospheric nightglow.
You should dilute the alkali too Moreover students who are just learning to experiment with acids for the first time may not be very careful. Undiluted acids may hurt them as they may. The results show that dilute alkali was the suitable pretreatment method and the conditions were first to soak the substrate with 1 sodium hydroxide with a solid-liquid ratio of 110 at 40 degrees C for 24 h and then subjected to 121 degrees C for 30 min.
Acids bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali.
Hydrolysis using dilute alkali. This is the usual way of hydrolyzing esters. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution.
There are two advantages of doing this rather than using a dilute acid. The reactions are one-way rather than reversible and the products are easier to separate. Biotransformation of lignin to lipids is challenging due to lignins recalcitrant nature as a phenolic heteropolymer with a nonuniform structure that imparts rigidity and recalcitrance of plant cell walls.
In this study wild and engineered Rhodococcus strains R. Opacus PD630 and R. Jostii RHA1 VanA with lignin degradation andor lipid biosynthesis capacities were selected to establish.
Oppositely treatment with dilute alkali has shown lower performances under the conditions explored most likely given the relatively significant lignin content suggesting that the use of stronger alkali regime with the associated drawbacks is unavoidable to improve the performance of this treatment. A dilute acid is that in which the concentration of the water mixed in the acid is higher than the concentration of the acid itself. For instance 5 sulfuric acid is a dilute acid.
A dilute acid unlike a concentrated acid will ionize to a greater degree in their solution higher percent dissociation with decreasing concentration. In the aldol condensation of acetaldehyde and acetone in dilute alkali the carbanion source will be. Dilute solutions of alkali metals in liq.
Ammonia are dark blue in colour due to solvated metal ions and solvated electrons. This solution is highly conductive due to the presence of solvated ions and electrons. Answer verified by Toppr Upvote 0.