Thus aliphatic compounds are the opposite of aromatic compounds. Aliphatic from Greek aleiphar fat described hydrocarbons derived by chemical degradation of fats or oils.

There were no water-based paints which of course thin with water.
What is aliphatic hydrocarbon. An aliphatic compound is a hydrocarbon compound containing carbon and hydrogen joined together in straight chains branched trains or non-aromatic rings. Aliphatic compounds may be saturated eg hexane and other alkanes or unsaturated eg hexene and other alkenes as well as alkynes. The simplest aliphatic hydrocarbon is methane CH 4.
Aliphatic hydrocarbons belong to the most abundant fraction in crude oil. Aliphatics molecules are linear or branched open-chain structures such as n -alkanes isoalkanes cycloalkanes naphthenes terpenes and steranes. It refers to hydrocarbons obtained by the chemical degradation of oils or fats.
An aliphatic compound is an organic one. It has carbon and hydrogen joined together in straight branched chains or non-aromatic rings. These compounds may be both saturated alkanes and.
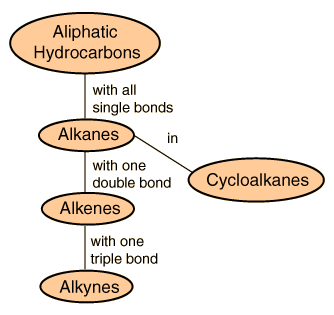
Aliphatic hydrocarbons are hydrocarbons based on chains of C atoms. There are three types of aliphatic hydrocarbons. Alkanes are aliphatic hydrocarbons with only single covalent bonds.
Alkenes are hydrocarbons that contain at least one CC double bond and a lkynes are hydrocarbons that contain a CC triple bond. Put these two terms together and you have the definition for an aliphatic hydrocarbon - a carbon-based compound that is straight branched or non-aromatic. An aliphatic compound may be a.
Aliphatic hydrocarbons include naphtha and mineral spirits which is also called paint thinner because when these solvents were introduced to replace turpentine for thinning paints all paints were oil-based. There were no water-based paints which of course thin with water. Aromatic hydrocarbons include benzene toluene and xylene.
25 rows Aliphatic compound. In organic chemistry hydrocarbons compounds composed solely. Aliphatic hydrocarbons are organic compounds composed of carbon and hydrogen atoms arranged in straight chains branched or non-aromatic ring structures.
Carbon and hydrogen atoms are bonded to each other via covalent bonds. Aliphatic hydrocarbons can be found in three types as alkanes alkenes and alkynes. Aliphatic hydrocarbon one in which no carbon atoms are joined to form a ring.
Aromatic hydrocarbon one that has cyclic structure and a closed conjugated system of double bonds. Chlorinated hydrocarbon any hydrocarbon compound with chlorine substitutions. The hydrocarbons of the alkane alkene and alkyne series are aliphatic compounds as are fatty acids and many other compounds.
Most compounds containing rings are aromatic compounds. Thus aliphatic compounds are the opposite of aromatic compounds. Aliphatic from Greek aleiphar fat described hydrocarbons derived by chemical degradation of fats or oils.
Aromatic hydrocarbons constituted a group of related substances obtained by chemical degradation of certain pleasant-smelling plant extracts. Aliphatic Hydrocarbons are hydrocarbon compounds containing Carbon and Hydrogen linked together with straight branched chains or non-aromatic rings. They can be either saturated butane pentane or other alkanes or unsaturated eg.
Propene or other alkenes as well as alkynes. In other settings aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids alkalis most oxidizing agents and most reducing agents.
When heated sufficiently or when ignited in the presence of air oxygen or strong oxidizing agents they burn exothermically to produce carbon dioxide and water. This organic chemistry video tutorial provides a basic introduction into hydrocarbons. It explains the difference between aliphatic hydrocarbons and aromati.
An aliphatic compound is a compound containing carbon and hydrogen joined together in straight chains branched trains or non-aromatic rings. These compounds are used as corrosion inhibitors. The hydrocarbons of the alkane alkene and alkyne series are aliphatic compounds as are fatty acids and many other compounds.