Strategies in Enzyme Catalysis Once a substrate has been bound it is the enzymes job to quickly transform the substrate into product. In this confirmation the substrates are forced into a reactive state.

COVALENT CATALYSIS The process of covalent catalysis involves the formation of a covalent bond between the enzyme and one or more substrates.
What is involved in enzyme catalysis. Some of these include. Enzymes can destabilize bonds within the substrate. Conformational changes in the enzyme upon substrate binding can bring reactive groups closer.
Proton donors and acceptors. The presence of acidic or basic groups can affect bond. Enzymes catalyze biochemical reactions.
They are similar to other chemical catalysts in many ways. Enzymes and chemical catalysts both affect the rate but not the equilibrium constant of a chemical reaction. Reactions proceed downhill energetically in accord with the Second Law of Thermodynamics.
Enzyme Catalysis - An enzyme is a substance which fastens a chemical reaction. A substrate is attracted towards the active site of the enzyme which leads to the catalysis of a chemical reaction and formation of products. Read more about the.
Enzyme catalysis is the primary activity in energy and information metabolism and enzyme cofactors are key to the catalytic ability of most enzymes. Pyridoxal 5-phosphate PLP cofactor derived from Vitamin B6 is widely distributed in nature and has significant latitude in catalytic diversity. In enzyme catalysis enzymes bind its substrate s it changes conformation and forces the substrates into a strained or distorted structure that resembles the T-State.
For example the enzyme hexokinase closes like a clamshell when it binds glucose. In this confirmation the substrates are forced into a reactive state. Strategies in Enzyme Catalysis Once a substrate has been bound it is the enzymes job to quickly transform the substrate into product.
The enzyme does so by carrying the substrate over a catalytic pathway. In a catalytic pathway the reaction takes a different course than it would on its own. Enzyme Catalysis Introduction.
In general enzymes are proteins produced by living cells they act as catalysts in biochemical reactions. A catalyst affects the rate of a chemical reaction. One consequence of enzyme activity is that cells can carry out complex chemical activities at relative low temperatures.
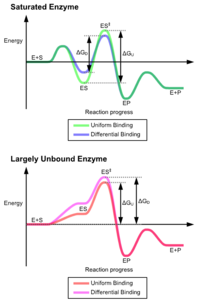
In an enzyme-catalyzed reaction the substance to be. Continue reading Enzyme. When enzyme catalyzed solid line the enzyme binds the substrates ES then stabilizes the transition state ES to reduce the activation energy required to produce products EP which are finally released.
Enzymes are a class of catalysts that are responsible for facilitating and increasing the rate of many vital biochemical reactions in plants and animals. The catalysis in which enzymes act as a catalyst is called enzyme catalysis. Enzymes are complex compounds containing nitrogen.
Animals and plants produce these compounds naturally in their bodies. In the induced-fit theory of enzyme-substrate binding a substrate approaches the surface of an enzyme step 1 in box A B C and causes a change in the enzyme shape that results in the correct alignment of the catalytic groups triangles A and B. Circles C and D represent substrate-binding groups on the enzyme that are essential for catalytic activity.
Coenyzmes are biomolecules that provide chemical groups that help catalysis. Like enzymes themselves coenzymes are not changed during catalysis. This distinguishes them from other substrates such as ATP which are changed by enzyme action.
Coenzymes however are not made of. COVALENT CATALYSIS The process of covalent catalysis involves the formation of a covalent bond between the enzyme and one or more substrates. Covalent catalysis accelerates reaction rates through the transient formation of a catalyst substrate covalent bond.
Usually this covalent bond is formed by the reaction of a nucleophilic group on the catalyst with an electrophilic group on the. The acid- and base-catalyzed reactions at high and low pH are not directly relevant to catalysis by enzymes. They typically involve activation of the substrate by the addition or removal of a proton in a rapid pre-equilibrium followed by the rate-determining reaction of the conjugate acid or base.
Mechanism of Acid-base Catalysis. Enzymes are proteins that accelerate biochemical transformations by lowering the activation energy of reactions. Enzyme catalysis is the increase in the rate of a chemical reaction by the active site of a protein.
The protein catalyst enzyme may be part of a multi-subunit complex andor may transiently or permanently associate with a Cofactor. A fundamental task of proteins is to act as enzymescatalysts that increase the rate of virtually all the chemical reactions within cells. Although RNAs are capable of catalyzing some reactions most biological reactions are catalyzed by proteins.
In the absence of enzymatic catalysis most biochemical reactions are so slow that they would not occur under the mild conditions of temperature. Electrostatic catalysis occurs when the enzyme active site stabilizes the transition state of the reaction by forming electrostatic interactions with the substrate. The electrostatic interactions can be ionic ionic-dipole dipole-dipole or hydrophobic interactions.