It strongly stimulates the human respiratory tract causing sore throat chest tightness coughing bronchitis and. Readily detectable by most normal persons.
Wt typically around 10 wt meaning that the ozone gas is 10 percent of the total weight of the gas mixturegNm3.
What is ozone concentration. The ozone is a very good absorber of ultraviolet-B UVB wavelengths of radiation known to promote cancer in humans and many animals. Ozone is constantly reacting with other particles and then is regenerated during the day maintaining a constant ozone concentration. The amount is very small measured at a few parts per billion parts of air but important for UVB protection.
Ozone concentrations refer to the quantity of ozone O3 molecules in the air. In the Bay Area ozone is measured by numerous monitoring stations that record hourly concentrations at each site. These hourly data are used to calculate eight-hour peak ozone levels.
Ozone concentrations are strongly influenced by weather which likely contributes to the high variability over time and peaks such as in the hot sunny summers of 2003 2006 2018 2019 and 2020. Hazards of excessive ozone concentration. It strongly stimulates the human respiratory tract causing sore throat chest tightness coughing bronchitis and.
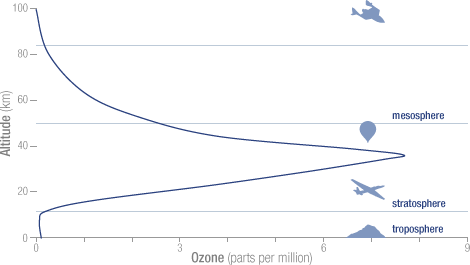
Ozone can cause nerve poisoning dizziness headache vision loss and memory loss. Ozone can destroy vitamin E in human skin. The vast majority of ozone sits in the stratosphere an atmospheric layer between six and 31 miles 10 to 50 kilometers above our planets surface.
Ozone makes up roughly 000006 of the. Could you provide a brief overview into Ozone concentration. Wt typically around 10 wt meaning that the ozone gas is 10 percent of the total weight of the gas mixturegNm3.
The conversions between both these units are given and they are pretty much the same. Ozone concentration is the ratio of total feed-gas to ozone production. As flow rate of feed-gas through an ozone generator decreases ozone concentration increases.
As flow increases less of the oxygen in the feed-gas is converted into ozone as the contact time for the feed-gas in the ozone generator decreases there is less time to produce ozone out of the given volume of gas. Hence indoor ozone concentrations can vary significantly from hour-to-hour day-to-day and season-to-season as well as from room-to-room and structure-to- structure. Under normal con ditions the half-life of ozone indoors is between 7 and 10 min and is determined primarily by surface removal and air exchange.
Al though reactions between ozone and most other indoor pollutants are. In the lower atmosphere the troposphere near the Earths surface ozone is created by chemical reactions between air pollutants from vehicle exhaust gasoline vapors and other emissions. At ground level high concentrations of ozone are toxic to people and plants.
The threshold of odor perception by the average person in clean air. Readily detectable by most normal persons. These concentrations can be measured with fair accuracy.
Ozone levels measured in typical residences and offices equipped with a properly operating electronic air cleaner when outdoor ozone level is. Ozone O3 is a colorless gas at ambient temperature and pressure and has a characteristic odor even at very low concentrations. It has a strong oxidizing ability that is hazardous to plants and animals and is unstable especially at higher concentrations.
The Dobson Unit is the most common unit for measuring ozone concentration. One Dobson Unit is the number of molecules of ozone that would be required to create a layer of pure ozone 001 millimeters thick at a temperature of 0 degrees Celsius and a pressure of 1 atmosphere the air pressure at the surface of the Earth. The ozone layer or ozone shield is a region of Earths stratosphere that absorbs most of the Suns ultraviolet radiation.
It contains a high concentration of ozone O 3 in relation to other parts of the atmosphere although still small in relation to other gases in the stratosphereThe ozone layer contains less than 10 parts per million of ozone while the average ozone concentration in Earth. Ozone O3 is a relatively unstable molecule made up of three atoms of oxygen O. Although it represents only a tiny fraction of the atmosphere ozone is crucial for life on Earth.
The low ozone levels in this image are transparent blue while high levels are opaque white. Ozone is a chemical consisting of three oxygen atoms bonded in a V-like shape. It occurs both naturally through reactions in the atmosphere and as a pollutant released by transport and industrial activities.