It dissolves easily in water. Ferric nitrate nonahydrate CAS Number 7782-61-8.

PubChem Substance ID 24855367.
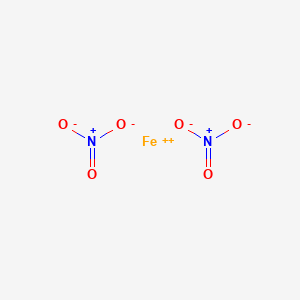
What is the formula for iron iii nitrate. Iron III Nitrate Iron III Nitrate Formula and Structure. The chemical formula of iron iii nitrate is and its molecular formula is. Occurrence of Iron III Nitrate.
Iron iii nitrate is deliquescent means it attract and hold water molecules either via. Preparation of Iron III Nitrate. Iron III Nitrate Structural Formula.
The structural representation of Iron III Nitrate is as shown in the figure below. Iron III Nitrate compound is obtained when the iron metal powder is treated with nitric acid. Fe 4 HNO 3 FeNO 3 3 NO 2 H 2 O.
To learn more about various chemical compounds and facts stay tuned with BYJUS. Iron III nitrate Main ChemSpider page Molecular formula. H 18 FeN 3 O 18 Molar mass.
403997 CAS Registry Number. Iron III nitrate nonahydrate ACS 980-1010. Iron III nitrate nonahydrate 98 metals basis.
IronIII nitrate nonahydrate 9995 trace metals basis Synonym. Ferric nitrate nonahydrate CAS Number 7782-61-8. Linear Formula FeNO 3 3 9H 2 O.
Molecular Weight 40400. PubChem Substance ID 24855367. Answer 1 of 4.
Iron nitrate reacts with sodium hydroxide to form iron hydroxide and sodium nitrate. The ionic equations for the reaction areFeNO33 —– Fe3 3NO3-1NaOH —– Na1 OH-1The net ionic equation isFe3 3OH-1 3Na1 3NO3-1 —– FeOH3 3NaNO3The balanced equation for the reaction isFeNO33 3NaOH —– FeOH3 3NaNO3. IronII Nitrate has a formula of FeNO32 From valencies.
FeII has an oxidation state of 2 its valency is 2. NO3- has a valency of -1. Fe 2 FeNO32 is how the reaction will be at the end FeII has an oxidation state of 2 its valency is 2.
IronII nitrate is the nitrate of iron that is a green solid and is unstable to heat at room temperature. When heated it is oxidized to IronIII nitrate. It is prepared by reacting iron metal with cold dilute nitric acid which results in the green hexahydrate if it is done below -10 C it forms the nonahydrate which decomposes back into the hexahydrate when returned to room temperature.
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. IronIII nitrate or ferric nitrate is the chemical compound with the formula FeNO33. IronIII nitrate also known as ferric nitrate is a chemical compound.
Its chemical formula is FeNO 3 3. It contains iron in its 3 oxidation state. It also contains nitrate ions.
IronIII nitrate is a light purple solid. It dissolves easily in water. It is an oxidizing agent.
It absorbs water easily to make a hydrate chemical with water molecules attached to it. The nitrate anion is a univalent -1 charge polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms Formula. NO 3 for a total formula weight of 6205.
IronII Nitrate molecular weight. Molar mass of FeNO32 1798548 gmol. Convert grams IronII Nitrate to moles or moles IronII Nitrate to grams.
55845 140067 15999432 Percent composition by element. What is the chemical formula for mercury II nitrate. What is the chemical formula for sodium chloride.
What is the chemical formula for barium nitride. What is the formula for ammonium nitrate. What is the formula for calcium bromide.
What is the chemical name for PbCr2O72. What is the chemical name for Fe2O3. Iron III oxide.
Chemical Elements Periodic Table Compound Name Formula Search Moles to Grams Calculator Common Compounds List Chemical Equation Balancer Complete List of Acids Complete List of Bases Molar to Mass Concentration Converter Molar. The correct formula for Lead IV nitrate is. The correct name for P2O5.
What is the formula for xenon tetrafluoride. The formula for calcium hydrogen sulfate is. The correct formula for iron III phosphide is.
The correct formula for bromic acid. HBrO3aq The correct name for aqueous solution of H2CO3 is. What is the.
IronIII nitrate also known as ferric nitrate is a chemical compound. Its chemical formula is FeNO 3 3. It contains iron in its 3 oxidation state.
It also contains nitrate ions. IronIII nitrate is a light purple solid. It dissolves easily in water.
It is an oxidizing agent.