Generally it is not the actual metal structure being studied. The counter electrode also known as auxiliary electrode is an electrode which is used to close the current circuit in the electrochemical cell.
The working electrode is a key factor in the process directing the course of the electrochemical reaction according to its properties.
What is working electrode. In corrosion testing the working electrode is a sample of the corroding metal. Generally it is not the actual metal structure being studied. Instead a small sample is used to represent the structure.
The working electrode can be bare metal or coated. The working electrode can be referred to as either cathodic or anodic. The working electrode is a key factor in the process directing the course of the electrochemical reaction according to its properties.
Material adsorbent surface etc. The working electrode must be stable towards corrosion and may be improved by additives or surface treatments. Electrode types The working electrode WE represents the most important component of an electrochemical cell.
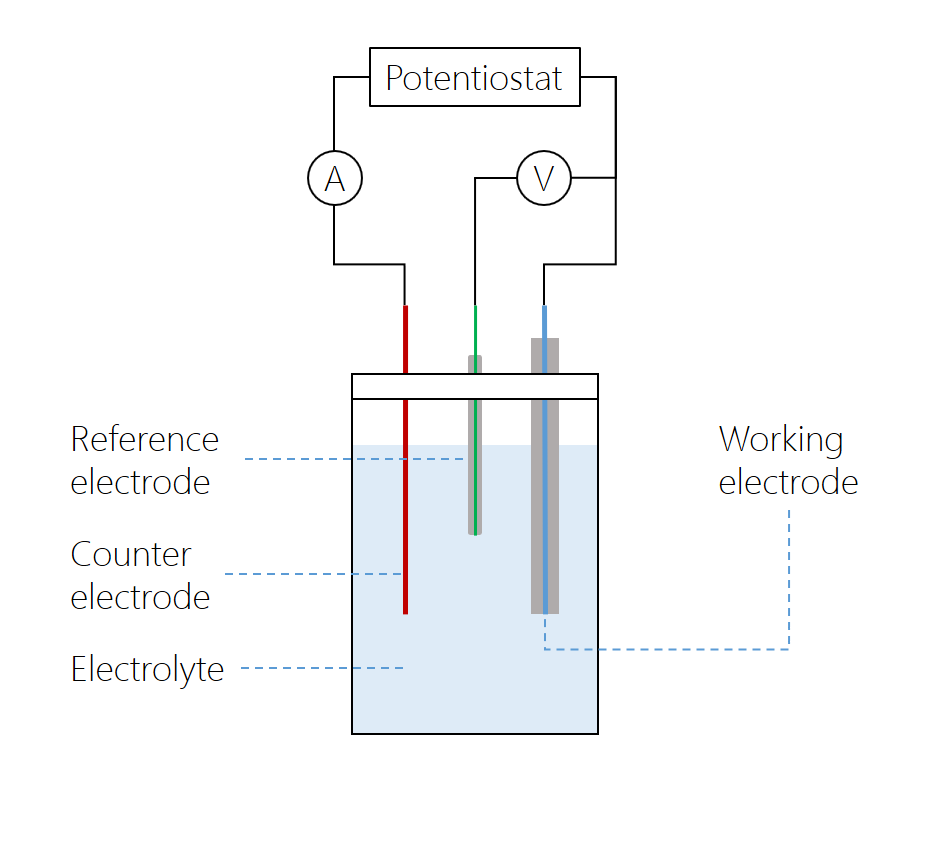
Advantages and limitations Table 1 lists the commonly used electrode materials and summarizes the advantages and. A working electrode WE is also called an indicator electrode it is used to reveal the properties of the analyte solution. In a 2-electrode cell you measure cell potential E between a WE and a reference electrode RE using a high impedance v.
Working Electrodes Redox Reactions. Redox reactions also referred to as oxidation-reduction reactions involve the loss or gain of. All dimensions are in millimetres.
For L-shaped and net platinum and gold electrodes please contact us. Platinum Disc Working. Working electrodes This is where it all happens.
Working electrodes are of utter importance because this is where everything that interests the electrochemist will happen. A large offer of working electrodes are available with a very wide range of size and materials. Working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring.
Common working electrodes can be made of inert materials such as Ag AuPt glassy carbonGC and Hg drop and film electrodes etc. For corrosion applications the material of the working electrode is the material under. Working electrode is the designation for the electrode being studied.
In corrosion experiments this is probably the material that is corroding. In physical-electrochemistry experiments this is most often an inert materialcommonly gold platinum or carbonwhich passes current to other species without being affected by that current. The counter electrode also known as auxiliary electrode is an electrode which is used to close the current circuit in the electrochemical cell.
It is usually made of an inert material eg. When a three electrode cell is used to perform electroanalytical chemistry the auxiliary electrode along with the working electrode provides a circuit over which current is either applied or measured. Here the potential of the auxiliary electrode is usually not measured and is adjusted so as to balance the reaction occurring at the working electrode.
This configuration allows the potential of the working. The platinum counter electrode Figure 3C was prepared by cyclic voltammetry using three electrodes which are the FTO working electrode AgAgCl reference electrode and platinum counter electrode at a scan rate of 10 mV ssup-1 with a supporting electrolyte prepared by Ksub2Pt Clsub6 1x 10sup-4 mol Lsup-1 dissolved in 01 mol Lsup-1 of HCl. The purpose of the auxiliary electrode AE is to provide a pathway for current to flow in the electrochemical cell without passing significant current through the reference electrode.
There are no specific material requirements for the electrode beyond it not adversely influencing reactions occurring at the working electrode WE. Precisely the electrical potential of an electrode working electrode WE or measuring electrode with reference to a second electrode which does not pass any. In a 3 electrode system current flows between the working electrode WE and counter electrode CE.
That entails a potential drop between those two electrodes. Ideally no current flows through the. The working electrode is where the reaction of interest happens eg.
Copper ions reduced to solid copper. The auxiliarycounter electrode supplies the current to the working electrode ie. It is the source of the electrons maybe not in all cases eg.
Zinc metal is oxidised to zinc ions. The reference electrode lets you control the potential difference so you dont apply too much driving. The counter or auxiliary electrode The counter or auxiliary electrode provides a means of applying input potential to the working electrode.
The purpose of these electrodes is. The working electrode consists of a carbon or metal rod embedded in a plastic block. Glassy carbon is the most common electrode for LCEC.
Specialty materials such as platinum gold silver nickel and carbon paste are used for specific assays described in BASi applications capsules. The block is made of PEEK polyetheretherketone and is resistant to solvents and. Simple Versatile Effective Electrode welding also known as manual arc welding manual electrode welding manual metal arc welding or shielded metal arc welding SMAW is usually the first welding process that welders are taught during training.
