Es gibt drei primäre Phasen der Materie. In the same manner at room temperature heat.

When temperature or pressure increases molecules interact more with each other.
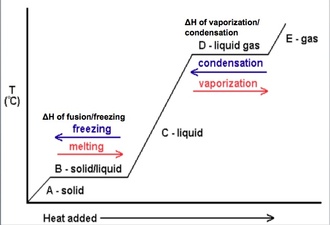
What phase changes are exothermic. There are six changes of phase that substances go through. Fusion or melting when a solid transforms to liquid. Vaporization when a liquid transforms to gas.
The following are exothermic phase changes. Freezing when a liquid transforms to solid. Condensation when a gas transforms to liquid.
In endothermic phase changes intermolecular binds are broken requiring energy. Exothermic An exothermic phase change releases heat energy into its environment. These changes include freezing and condensation.
When a substance loses heat energy the attractive forces between atoms slow them down reducing their mobility. For this to happen heat must leave the substance such as water turning into ice cubes in your freezer. In the same manner at room temperature heat.
Es gibt drei primäre Phasen der Materie. Fest flüssig und gasförmig. Ein Feststoff der flüssig wird wird Schmelzen oder Schmelzen genannt.
Ein Feststoff der gasförmig wird wird Sublimation genannt. Eine flüssig werdende Flüssigkeit wird als Gefrieren bezeichnet. Change of phase from gas to liquid to solid are exothermic reaction.
Condensation deposition and freezing are exothermic processes that undergo change in phase. 2 question What are the names of the phase changes which are exothermic. Also Know which change is exothermic.
Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Changes of state are examples of phase changes or phase transitions. All phase changes are accompanied by changes in the energy of a system.
Phase changes typically occur when the temperature or pressure of a system is altered. When temperature or pressure increases molecules interact more with each other. When pressure increases or temperature decreases its easier for atoms and molecules to.
As time passes and water temperature drops the gas molecules water vapor around that glass slow down and change state from gas to liquid as they collect on the surface of the glass. This is an exothermic reaction because heat is technically given off in order for the gas to cool and change state. Take a piece of metal and get it wet.
The exothermic phase changes are gas–. Looking at a phase diagram if you are going up the stairs the conversions require energy going down. Exothermic reactions These are reactions that transfer energy to the surroundings ie the energy exits from the reaction hence the name exothermic.
The energy is usually transferred as heat. These are known as exothermic. For purposes of this discussion processes that require or give off heat will be limited to changes of state known as phase changes and changes in chemical.
What is an exothermic change. The system that releases energy to its surroundings. What are the endothermic phase changes.
What are the exothermic phase changes. What is heat of fusion. The amount of energy required to change a solid to a liquid.
Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Changes of state are examples of phase changes or phase transitions. All phase changes are accompanied by changes.
Students will be able to explain the 6 phase changes and determine which phase changes are exothermic and which phase changes are endothermic. A phase change is a physical change. The three states of matter are solid liquid and gas.
1 -2 class periods.