Sulfur was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine both of which involved burning it to form sulfur dioxide and allowing this to be absorbed by wet clothes or the grape juice. Elemental sulfur is a bright yellow crystalline solid at room temperature.

Through its major derivative sulfuric acid sulfur ranks as one of the more-important elements used as an industrial raw material.
What state is sulfur. Sulfur is pale yellow odorless brittle solid which is insoluble in water but soluble in carbon disulfide. In every state whether gas liquid or solid elemental sulfur occurs in more than one allotropic form or modification. These present a confusing multitude of forms whose relations are not yet fully understood.
Sulfur was mined near Mount Etna in Sicily and used for bleaching cloth and preserving wine both of which involved burning it to form sulfur dioxide and allowing this to be absorbed by wet clothes or the grape juice. For centuries sulfur along with mercury and salt was believed to be a component of all metals and formed the basis of alchemy whereby one metal could be transmuted into another. Sulfur oxides are the oxides of sulfur with oxygen in -2 oxidation state.
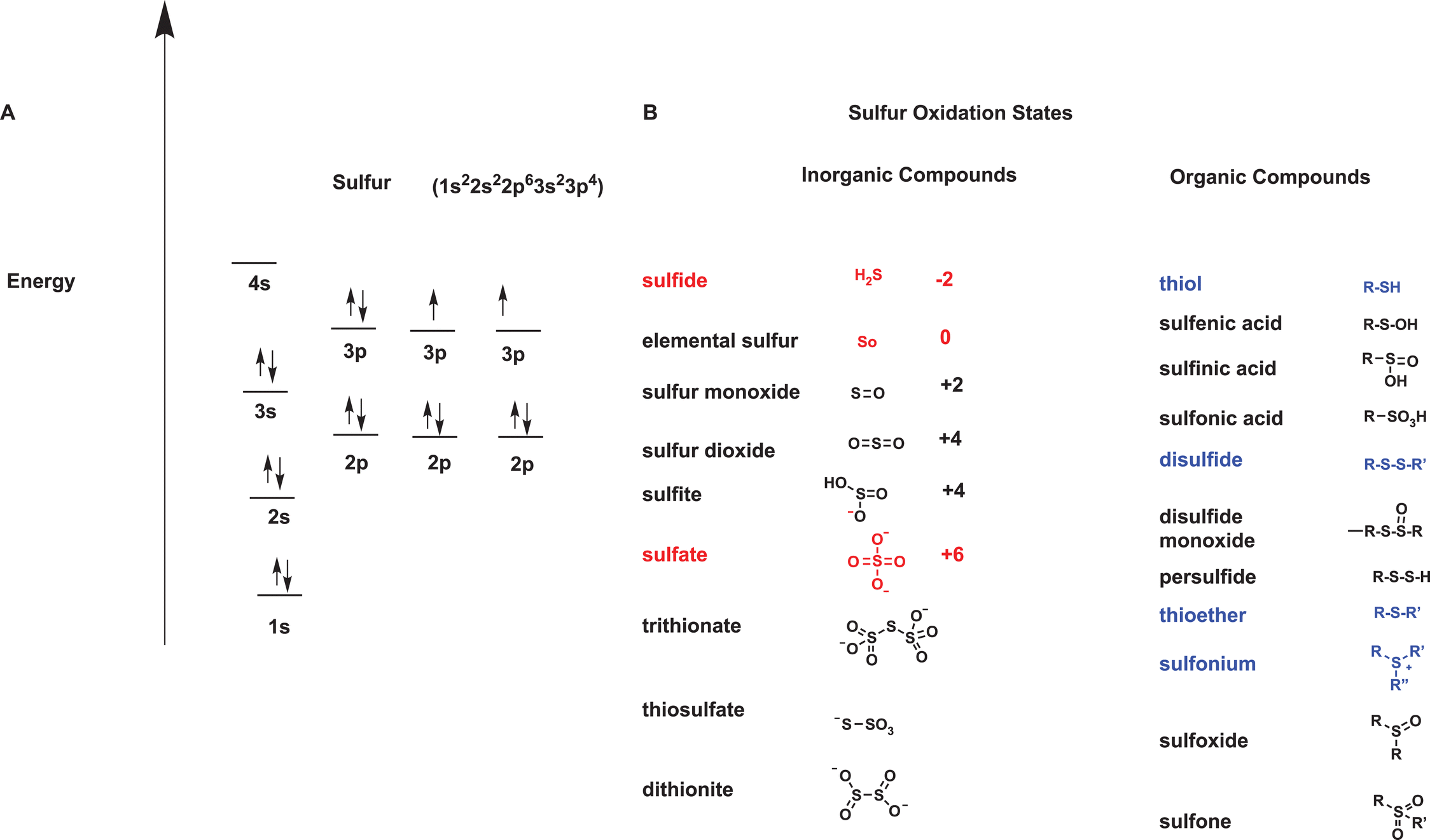
There are two main oxides of sulfur. They are sulfur dioxide SO2 and sulfur trioxide SO3. 2 1 Oxidation state of S 3 2 2 1 0.
2 Oxidation state of S 6 2 0. Hence oxidation state of S in H 2 SO 4 6. Sulfur Statistics and Information.
Through its major derivative sulfuric acid sulfur ranks as one of the more-important elements used as an industrial raw material. It is of prime importance to every sector of the worlds industrial and fertilizer complexes. Sulfuric acid production is the major end use for sulfur and consumption of sulfuric.
Sulfur is an element that exists in nature and can be found in soil plants foods and water. 1 Some proteins contain sulfur in the form of amino acids. 2 Sulfur is an essential nutrient for plants.
3 Sulfur can kill insects mites fungi and rodents. Sulfur has been registered for use in pesticide products in the United States since the 1920s. State of sulfur at 100.
Answered 4 years ago. Sonic the Hedgehog franchise Silver Sulfur Is silver tarnishing as the silver metal reacts with sulfur a chemical change. Answered 4 years ago.
Definitions Sulfur What are sulfur compounds. Answered 5 years ago. Sulfur Is sulfur magnetic.
Answered 5 years ago. Sulfur What is the valency of sulphur dioxide. Answered 5 years ago.
2 1 Oxidation state of S 4 2 0. Sulfur S also spelled sulphur nonmetallic chemical element belonging to the oxygen group Group 16 VIa of the periodic table one of the most reactive of the elements. Pure sulfur is a tasteless odourless brittle solid that is pale yellow in colour a poor conductor of electricity and insoluble in water.
What state is sulfur at room temperature. By signing up youll get thousands of step-by-step solutions to your homework questions. No atom can have a fractional oxidation state - they must be whole numbers you cant lose half of an electron.
As a consequence this compound must have sulfur atoms with mixed oxidation states. Since it is normal for sulfur to have oxidation states of -2 0 2 4 and 6 it is most likely that there are three sulfurs with a 2 oxidation state and one sulfur that is 4. The average of these is 25.
What is Sulfur. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The chemical symbol for Sulfur is S.
Sulfur is abundant multivalent and nonmetallic. Under normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow crystalline solid at room temperature.
Common oxidation states of sulfur range from 2 to 6. Sulfur forms stable compounds with all elements except the noble gases. For some organic sulfur compounds smell depends on their concentration.
The sulfur-containing monoterpenoid grapefruit mercaptan has the characteristic scent of grapefruit in small concentrations but has a unpleasant thiol odor at larger concentrations. Sulfur forms compounds in oxidation states 2 sulfide S 2 4 sulfite SO 32 and 6 sulfate SO 42. It combines with nearly all elements.
An unusual feature of some sulfur compounds results from the fact that sulfur is second only to carbon in exhibiting catenation ie the bonding of.