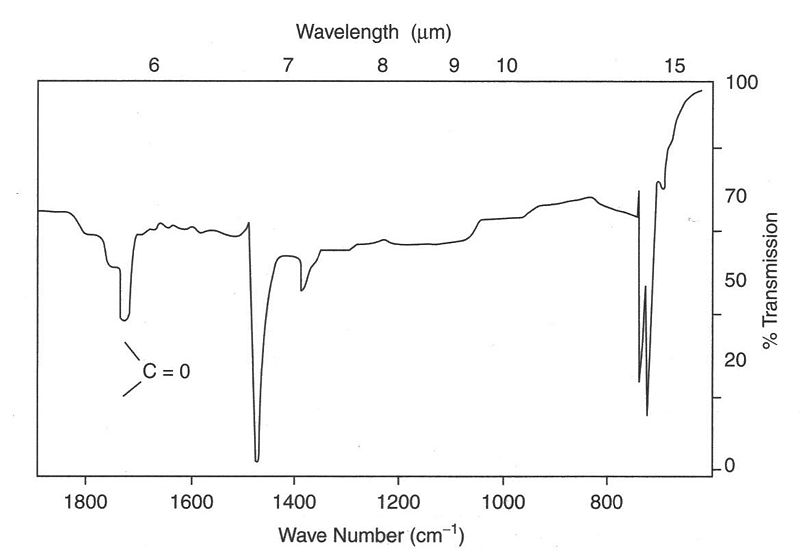
When molecules absorb IR radiation transitions occur from a ground vibrational state to an excited vibrational state. Vibrational spectroscopy is used for the identification of mineral phases based on their fingerprint patterns.

Infrared IR spectroscopy or vibrational spectroscopy is an analytical technique that takes advantage of the vibrational transitions of a molecule.
Why is infrared spectroscopy sometimes referred to as vibrational spectroscopy. In IR polychromatic light light having different frequencies is passed through a sample and the intensity of the transmitted light is measured at each frequency. When molecules absorb IR radiation transitions occur from a ground vibrational state to an excited vibrational state. For all those reason IR called Vibrational spectra.
The most widely used vibrational spectroscopy is Infrared IR spectroscopy. In IR spectroscopy an infrared lamp produces electromagnetic radiation between the wavelengths of 700 nm to 1 mm. Converting wavelength to frequency ν α 1λ is convenient for this type of spectroscopy because vibrational excitations occur at characteristic frequencies in molecules.
Vibrational spectroscopy is used for the identification of mineral phases based on their fingerprint patterns. Both Raman and infrared spectra which provide complementary information were used in early analyses to demonstrate that the mineral in OI bone was apatitic and suggest that it was smaller andor less crystalline than that in aged-matched controls. Vibrational Spectroscopy IR Raman Vibrational spectroscopy.
In order to describe the 3N-6 or 3N-5 different possibilities how non-linear and linear molecules containing N atoms can vibrate the models of the harmonic and anharmonic oscillators are used. These modes of vibration normal modes give rise to absorption bands IR if the sample is irradiated with. Infrared IR spectroscopy or vibrational spectroscopy is an analytical technique that takes advantage of the vibrational transitions of a molecule.
It is one of the most common and widely used spectroscopic techniques employed mainly by inorganic and organic chemists due to its usefulness in determining structures of compounds and identifying them. Infrared spectroscopy IR spectroscopy or vibrational spectroscopy involves the interaction of infrared radiation with inorganic chemicals and covers a range of techniques mostly based on absorption spectroscopy. As with all spectroscopic techniques it can be used to identify and study inorganic chemicals.
For a given sample which may be solid liquid or gaseous the method or technique of. Vibrational Infrared IR Spectroscopy It is the technique which is used to identify chemical compounds based on how infrared radiations are absorbed by the compounds chemical bonds and interacts with them. The most common technique used is absorption spectroscopy.
In infrared spectroscopy the sample is irradiated with polychromatic light and a photon of light is absorbed when the frequency energy of the absorbed light matches the energy required for a particular bond to vibrate within the sample. In order for a vibration to be infrared active the molecular dipole moment must change during the vibration. Infrared Spectroscopy is the analysis of infrared light interacting with a molecule.
This can be analyzed in three ways by measuring absorption emission and reflection. The main use of this technique is in organic and inorganic chemistry. It is used by chemists to determine functional groups in molecules.
IR Spectroscopy measures the vibrations of atoms and based on this it is possible to. Significant broadening can happen also in a gas if collisions are frequent due to high pressure in which case it is not surprisingly referred to as pressure broadening. Collisions shorten the lifetime of particular vibrational states leading to uncertainty in the energy and thereby frequency which translates into broadening.
A collisional model provides similar insight in a gas and a non-H. Infrared spectroscopy IR spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques mostly based on absorption spectroscopy.
As with all spectroscopic techniques it can be used to identify and study chemicals. A common laboratory instrument that uses. 100 1 rating IR spectroscopy is concerned with the study of absorption of infrared radiation which causes vibrational transition in the molecules Hence IR spectroscopy is called vibrational spectroscop view the full answer.
Previous question Next question. Vibrational Spectroscopy provides a vehicle for the publication of original research that focuses on vibrational spectroscopy. This covers infrared near-infrared and Raman spectroscopies and publishes papers dealing with developments in applications theory techniques and instrumentation.
The topics covered by the journal include. Infrared IR spectroscopy is one of the most common and widely used spectroscopic techniques employed mainly by inorganic and organic chemists due to its usefulness in determining structures of compounds and identifying them. Chemical compounds have different chemical properties due to the presence of different functional groups.
It is because the atmosphere of the exoplanets shows the most variable special features at infrared wavelength. Hence the astronomers can easily look for these features on. In practice infrared spectra do not normally display separate absorption signals for each of the 3n-6 fundamental vibrational modes of a molecule.
The number of observed absorptions may be increased by additive and subtractive interactions leading to combination tones and overtones of the fundamental vibrations in much the same way that sound vibrations from a musical instrument interact. This technique covers the region of the electromagnetic spectrum between the visible wavelength of 800 nanometres and the short-wavelength microwave 03 millimetre. The spectra observed in this region are primarily associated with the internal vibrational motion of molecules but a few light molecules.